Abstract
Introduction: The Reduced-dosed Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism (EINSTEIN CHOICE) trial found that continued anticoagulation therapy with rivaroxaban (10 mg or 20 mg) versus aspirin for up to 12 months after 6-12 months of initial anticoagulation significantly reduced the risk of recurrent venous thromboembolism (VTE) without significantly increasing bleeding rates. The current study aimed to assess the cost impact of rivaroxaban (assessed separately at 10 mg and 20 mg) vs. aspirin based on rates of recurrent VTE, all-cause mortality, and bleeding in the EINSTEIN CHOICE trial.
Methods: Total healthcare costs associated with rivaroxaban and aspirin were calculated as the sum of clinical event costs and drug costs from a US managed care perspective and converted to per patient per month (PPPM) costs. Clinical event costs for each treatment course were calculated by multiplying the event rate by the cost of care. One-year Kaplan-Meier clinical event rates for recurrent pulmonary embolism (PE), recurrent deep-vein thrombosis (DVT), all-cause mortality, major bleeding and clinically relevant non-major bleeding were obtained from the EINSTEIN CHOICE study for each treatment course (rivaroxaban 10 mg, rivaroxaban 20 mg, aspirin). The one-year cost of care for a patient with a clinical event compared to a patient without the event was determined by a targeted literature review. Drug costs were calculated as the product of drug price and duration of treatment. Drug prices for rivaroxaban and aspirin were based on 2016 wholesale acquisition costs.
The duration of treatment was calculated based on the intended duration of treatment in the EINSTEIN CHOICE trial. Differences in clinical event costs, drug costs, and total healthcare costs between patients receiving rivaroxaban vs. aspirin for continued anticoagulation therapy were calculated. To account for the uncertainties surrounding the cost model inputs and to examine the impact of model inputs on the cost model, a one-way sensitivity analysis was conducted where results were examined over a ±20% range of each cost component and ±1 standard deviation for differences in clinical event rates between patients treated with rivaroxaban vs. aspirin. A sensitivity analysis with a 15% discount for rivaroxaban was also conducted, as insurance payers in the US market frequently negotiate with pharmaceutical companies for rebates on drug purchases. All costs were inflated to 2016 using Consumer Price Index medical services data from Bureau of Labor Statistics.
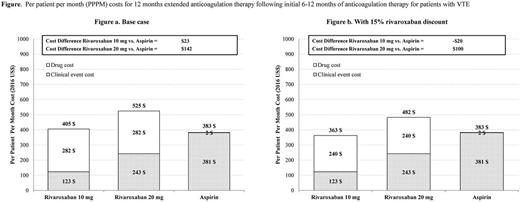
Results: The mean duration of treatment was 9.4 months for all treatment arms. Rivaroxaban users had lower PPPM clinical event costs compared with aspirin users ($123, $243, and $381 for rivaroxaban 10 mg, rivaroxaban 20 mg, and aspirin, respectively). However, the higher cost of rivaroxaban vs. aspirin made the total healthcare costs for patients treated with rivaroxaban exceed those treated with aspirin. Compared with aspirin treated patients, the PPPM total healthcare costs were $23 higher for patients treated with rivaroxaban 10 mg and $142 higher for those treated with rivaroxaban 20 mg (Figure). With a 15% discount for rivaroxaban 10 mg, the lower cost of clinical events for the rivaroxaban treated patients more than fully offset the higher drug costs, and yielded a $20 lower total healthcare cost. With a 15% discount for rivaroxaban 20 mg, the PPPM total healthcare costs remained higher but with a smaller difference (by $100) for patients treated with rivaroxaban vs. aspirin. The three model inputs with the greatest influence on total healthcare costs differences between rivaroxaban 10 mg and aspirin treated patients in the one-way sensitivity analysis were drug costs, all-cause mortality rate, and recurrent DVT rate.
Conclusion: Continued therapy with rivaroxaban 10 mg and 20 mg anticoagulation in VTE patients who had completed 6 to 12 months of initial anticoagulation therapy was associated with lower clinical event costs, but higher total costs, compared to aspirin, and rivaroxaban 10 mg was associated with lower total healthcare costs when a 15% drug cost discount was applied.
Davidson: Portola: Consultancy, Honoraria; Johnson & Johnson: Consultancy, Honoraria. Wells: Itreas: Other: Writing Committee; Janssen Scientific Affairs, LLC: Consultancy; BMS: Other: Grant Support; Pfizer: Other: Grant Support; Daiichi Sankyo: Other: Speaker Fees; Bayer Healthcare: Other: Speaker Fees, Ad Board Meeting, Research Funding. Prins: Janssen Scientific Affairs, LLC: Consultancy; Daiichi Sankyo: Consultancy; Pfizer: Consultancy. Beyer-Westendorf: Bayer: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Boehringer-Ingelheim: Honoraria, Research Funding. Lensing: Bayer Pharma AG: Employment. Haskell: Janssen Research & Development, LLC: Employment. Levitan: Zimmer Holdings, Inc.: Equity Ownership; Pharmaceutical Holdrs Trust: Equity Ownership; Baxter International, Inc.: Equity Ownership; Johnson & Johnson: Equity Ownership; Janssen Research & Development, LLC: Employment. Laliberté: Janssen Scientific Affairs, LLC: Research Funding. Ashton: Janssen Scientific Affairs, LLC: Employment. Xiao: Janssen Scientific Affairs, LLC: Research Funding. Lejeune: Janssen Scientific Affairs, LLC: Research Funding. Crivera: Janssen Scientific Affairs, LLC: Employment, Equity Ownership. Lefebvre: Janssen Scientific Affairs, LLC: Research Funding. Zhao: Janssen Scientific Affairs, LLC: Employment. Yuan: Janssen Research & Development, LLC: Employment. Schein: Janssen Scientific Affairs, LLC: Employment, Equity Ownership, Other: Own in excess of $10,000 of J&J stock. Prandoni: Pfizer: Consultancy; Sanofi: Consultancy; Bayer Pharma: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal